Author
Author  Correspondence author
Correspondence author
Journal of Vaccine Research, 2024, Vol. 14, No. 3
Received: 15 Apr., 2024 Accepted: 18 May, 2024 Published: 29 May, 2024
Hepatitis B virus (HBV) infection remains a significant global health challenge, with over 296 million people living with chronic HBV infection and more than 820 000 annual deaths due to liver cirrhosis and hepatocellular carcinoma (HCC). The epidemiology of HBV varies widely, with high endemicity in regions such as sub-Saharan Africa and East Asia, and lower prevalence in North America and Western Europe. Universal hepatitis B vaccination programs have been instrumental in reducing HBV prevalence and associated HCC rates, particularly in high-endemicity regions. Despite these successes, challenges remain, including variable vaccination coverage and the need for targeted strategies to address high-risk populations and regions with low immunization rates. Sustainable vaccination programs, improved coverage, and effective monitoring are essential to achieving the World Health Organization's goal of HBV elimination by 2030. This study highlights the global epidemiology of HBV, the impact of vaccination on disease burden, and the implications for future vaccine strategies to reduce HBV-related morbidity and mortality.
1 Introduction
Hepatitis B virus (HBV) is a significant global health concern, infecting the liver and potentially leading to chronic liver diseases such as cirrhosis and hepatocellular carcinoma (HCC) (Lavanchy, 2005; Shepard et al., 2006; Ott et al., 2012). HBV is highly infectious and can be transmitted through various routes, including vertical transmission from mother to child, sexual contact, household contact, and unsafe injections (Shepard et al., 2006). Chronic HBV infection is particularly prevalent in certain regions, with the highest endemicity observed in sub-Saharan Africa and East Asia (Ott et al., 2012; MacLachlan and Cowie, 2015). Despite the availability of effective vaccines and antiviral treatments, HBV remains a major cause of morbidity and mortality worldwide (Locarnini et al., 2015; Pattyn et al., 2021).
Understanding the global epidemiology of HBV is crucial for developing effective public health strategies and vaccine policies. Epidemiological studies provide insights into the prevalence, distribution, and transmission patterns of HBV, which are essential for identifying high-risk populations and regions (Ott et al., 2012; MacLachlan and Cowie, 2015; Razavi-Shearer et al., 2018). These studies also highlight the impact of vaccination programs and other preventive measures on reducing HBV prevalence and associated diseases (Shepard et al., 2006; Locarnini et al., 2015). For instance, the implementation of universal hepatitis B immunization has led to significant declines in chronic HBV infection rates in many countries, particularly among children (Shepard et al., 2006; Pattyn et al., 2021). However, the absolute number of chronically infected individuals continues to rise, underscoring the need for sustained and targeted interventions (Ott et al., 2012; Razavi-Shearer et al., 2018).
This study provides a comprehensive overview of the global epidemiology of HBV and its implications for vaccine strategies. It will examine the current prevalence and distribution of HBV infection, the effectiveness of existing vaccination programs, and the challenges in achieving global HBV control. By synthesizing data from multiple studies, this study seeks to inform public health policies and guide future research efforts to eliminate HBV as a public health threat by 2030.
2 Epidemiology of Hepatitis B
2.1 Global prevalence and incidence
Hepatitis B virus (HBV) infection remains a significant global health challenge, with an estimated 240 million people chronically infected worldwide (Ott et al., 2012; Lavanchy and Kane, 2016). The prevalence of chronic HBV infection varies significantly across different regions, with the highest endemicity observed in sub-Saharan Africa and East Asia, where prevalence rates can reach up to 8.6% (Ott et al., 2012). In contrast, regions such as North America, Western Europe, and parts of Latin America exhibit much lower prevalence rates, often below 2% (Ott et al., 2012; Razavi-Shearer et al., 2018). Despite a general decline in prevalence from 1990 to 2005, the absolute number of individuals living with chronic HBV infection has increased due to population growth, rising from 223 million in 1990 to 240 million in 2005 (Ott et al., 2012).
The global burden of HBV is further underscored by the high mortality associated with the infection, with approximately 800 000 deaths annually, primarily due to liver cancer and cirrhosis (Lavanchy and Kane, 2016). The World Health Organization (WHO) has recognized viral hepatitis as a critical public health issue, ranking HBV as the 15th leading cause of death globally (Lavanchy and Kane, 2016). Efforts to combat HBV have led to significant reductions in prevalence among younger populations, particularly in regions with robust vaccination programs (Lavanchy and Kane, 2016).
2.2 Modes of transmission
HBV is transmitted through parenteral or mucosal exposure to infected blood and body fluids. The primary modes of transmission include vertical transmission from mother to child during childbirth, horizontal transmission through close household contact, sexual contact, and unsafe medical practices such as unsterilized injections and blood transfusions (Shepard et al., 2006; Te and Jensen, 2010; Hwang et al., 2012). In highly endemic areas, vertical and early childhood horizontal transmission are predominant, leading to a high rate of chronic infection (Hwang et al., 2012). Conversely, in low endemic regions, transmission typically occurs in adulthood, often resulting in acute, self-limiting infections (Hwang et al., 2012).
The risk of chronic infection is significantly higher when HBV is acquired during infancy or early childhood, with chronicity rates of up to 90% in perinatal infections compared to less than 5% in adult-acquired infections (Shepard et al., 2006). This highlights the critical importance of preventing mother-to-child transmission through timely birth-dose vaccination and the use of hepatitis B immunoglobulin (Shepard et al., 2006; Razavi-Shearer et al., 2018).
2.3 Regional variations
The epidemiology of HBV infection exhibits considerable regional variation, influenced by factors such as vaccination coverage, healthcare infrastructure, and public health policies. In sub-Saharan Africa and East Asia, high endemicity rates are observed, with significant public health efforts focused on reducing mother-to-child transmission and improving vaccination coverage (Ott et al., 2012; Razavi-Shearer et al., 2018). In these regions, the implementation of universal infant vaccination programs has led to substantial declines in HBV prevalence among children (Lavanchy and Kane, 2016; Razavi‐Shearer et al., 2018).
In Latin America, the pattern of HBV endemicity varies widely, with low prevalence in temperate South America and parts of the Caribbean, moderate prevalence in Brazil and Andean countries, and high prevalence in the Amazon basin (Oh, 1990). Strategies to combat HBV in this region emphasize the prevention of perinatal and early childhood transmission, alongside targeted vaccination of high-risk adult populations (Oh, 1990). In North America and Western Europe, HBV prevalence is generally low, attributed to effective vaccination programs, stringent blood screening practices, and public health education (Ott et al., 2012). However, these regions face challenges related to migration from high-prevalence countries, necessitating tailored public health responses to address the needs of immigrant populations (Locarnini et al., 2015).
The global epidemiology of HBV is shaped by a complex interplay of demographic, socio-economic, and healthcare factors. Continued efforts to enhance vaccination coverage, improve diagnostic and treatment access, and implement targeted public health interventions are essential to reduce the burden of HBV and achieve the WHO's goal of eliminating viral hepatitis as a public health threat by 2030 (Locarnini et al., 2015; Razavi‐Shearer et al., 2018).
3 Health Impact of Hepatitis B
3.1 Acute vs. chronic infection
Hepatitis B virus (HBV) infection can manifest in two primary forms: acute and chronic. Acute HBV infection is typically a short-term illness that occurs within the first six months after exposure to the virus. Symptoms can range from mild to severe and may include jaundice, fatigue, abdominal pain, and nausea. In most healthy adults, the immune system can clear the virus, leading to complete recovery without long-term health issues. However, in some cases, acute infection can lead to fulminant hepatitis, a rare but severe form of liver failure that can be fatal (Chen and Gluud, 2005; Zanetti et al., 2008).
Chronic HBV infection, on the other hand, occurs when the virus remains in the body for more than six months. This is more likely to happen if the infection is acquired at birth or during early childhood, as the immune system is less likely to clear the virus at these stages. Chronic HBV can lead to serious health problems, including cirrhosis (scarring of the liver), liver failure, and hepatocellular carcinoma (HCC), a type of liver cancer. Chronic infection is a significant global health issue, with an estimated 360 million people living with chronic HBV worldwide (Figure 1) (Shepard et al., 2006; Ott et al., 2012; Gomes et al., 2019).
|
Figure 1 Prevalence of Hepatitis B Infection in Children Under 5 and Adults Aged 10-19 by Region, 2005 (Adapted from Ott et al., 2012) Image caption: This map illustrates the prevalence of hepatitis B virus (HBsAg) infection in different regions among children under 5 years old and adults aged 10-19 years in 2005; The colors represent different prevalence levels: light yellow (<2%, low), yellow (2%-4.9%, low-intermediate), orange (5%-7.9%, intermediate-high), and red (≥8%, high); The map shows that Africa and parts of Asia have high prevalence rates of hepatitis B, especially Central and Western Africa, where both children and adult infection rates exceed 8%; In contrast, North America, South America, Europe, and Oceania have lower infection rates, with most areas having a prevalence of less than 2%; These regional differences in prevalence may be related to factors such as vaccine coverage, healthcare conditions, and public health policies; This information is crucial for developing and implementing vaccination strategies and public health policies (Adapted from Ott et al., 2012) |
3.2 Morbidity and mortality
The morbidity and mortality associated with HBV are substantial. Chronic HBV infection is a leading cause of liver-related morbidity and mortality globally. Each year, approximately 600 000 people die from HBV-related liver diseases, including cirrhosis and HCC (Goldstein et al., 2006; Shepard et al., 2006). The progression from chronic HBV infection to cirrhosis and HCC is a major concern, as these conditions significantly reduce life expectancy and quality of life.
The burden of HBV-related morbidity and mortality is not evenly distributed across the globe. Regions with high HBV endemicity, such as sub-Saharan Africa and East Asia, experience higher rates of chronic infection and related complications. For instance, in East Asia, the prevalence of HBsAg (a marker of chronic HBV infection) can be as high as 8.6% (Ott et al., 2012). The implementation of universal vaccination programs has led to significant reductions in HBV-related morbidity and mortality in many regions. For example, in Taiwan, the prevalence of chronic HBV infection in children has declined by more than 90% following the introduction of universal vaccination (Kao and Chen, 2002; Shepard et al., 2006).
3.3 Economic and social burden
The economic and social burden of HBV is profound. The direct medical costs associated with managing chronic HBV infection, cirrhosis, and HCC are substantial. These costs include expenses for antiviral treatments, liver transplantation, and ongoing medical care. In addition to direct medical costs, there are significant indirect costs related to lost productivity, as individuals with chronic HBV may experience reduced work capacity and early mortality (Locarnini et al., 2015; Hutin et al., 2018).
The social burden of HBV extends beyond the affected individuals to their families and communities. Stigma and discrimination against individuals with HBV can lead to social isolation and mental health issues. In many regions, particularly those with high HBV prevalence, there is a lack of awareness and understanding about the disease, which can hinder efforts to prevent and manage HBV infection effectively (Locarnini et al., 2015; Pattyn et al., 2021).
Vaccination remains the most cost-effective strategy to reduce the economic and social burden of HBV. Universal vaccination programs have been shown to be highly effective in preventing new infections and reducing the prevalence of chronic HBV. For instance, the World Health Organization (WHO) recommends the inclusion of the hepatitis B vaccine in national immunization programs, which has led to significant reductions in HBV-related morbidity and mortality in many countries (Zanetti et al., 2008; Hutin et al., 2018). However, challenges remain in achieving high vaccination coverage, particularly in low-resource settings where economic and social barriers can impede the implementation of vaccination programs (Kao and Chen, 2002; Zanetti et al., 2008).
The health impact of hepatitis B is multifaceted, encompassing acute and chronic infections, significant morbidity and mortality, and substantial economic and social burdens. Effective vaccination strategies are crucial in mitigating these impacts and moving towards the global elimination of HBV as a public health threat. Continued efforts are needed to overcome the barriers to vaccination and ensure that all individuals, regardless of their geographic or socioeconomic status, have access to life-saving vaccines.
4 Current Vaccination Strategies
4.1 Vaccine development and types
The development of hepatitis B vaccines has been a cornerstone in the fight against hepatitis B virus (HBV) infection. The first licensed hepatitis B vaccine was derived from the purification of hepatitis B surface antigen (HBsAg) from the plasma of asymptomatic carriers. This was followed by the advent of recombinant DNA technology, which enabled the production of recombinant hepatitis B vaccines. These vaccines are highly effective, providing long-term protection against HBV infection in more than 90% of healthy individuals who complete the vaccination series (Zhao et al., 2020; Pattyn et al., 2021).
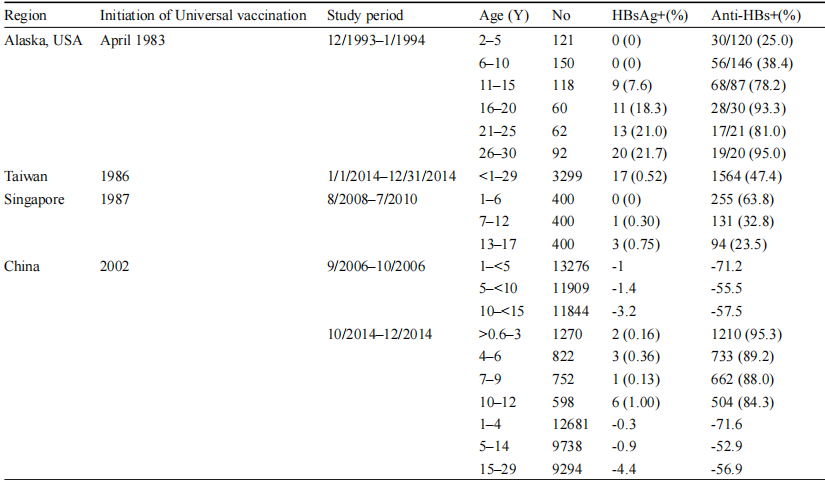
The standard hepatitis B vaccination schedule typically involves three doses, which can elicit long-term immunity lasting over 30 years. Additionally, the concurrent use of hepatitis B immunoglobulin (HBIG) and the hepatitis B vaccine has significantly reduced mother-to-child transmission (MTCT) of HBV. This combination has been particularly effective, reducing the infection rate to nearly zero in children born to HBsAg-negative mothers and to 5-10% in children born to HBsAg-positive mothers (Zhao et al., 2020). Despite these advancements, there are still challenges in vaccine implementation, particularly in regions with suboptimal vaccination coverage. Efforts are ongoing to improve the global reach of hepatitis B vaccination, especially in resource-poor settings where the burden of HBV is highest (Table 1) (Zhao et al., 2020; Pattyn et al., 2021).
|
Table 1 Prevalence of HBsAg and anti-HBs in children and young adults after universal vaccination in selected regions (Adopted from Zhao et al., 2020) Table caption: Table 1 shows the prevalence of Hepatitis B surface antigen (HBsAg) and anti-Hepatitis B surface antibody (anti-HBs) in children and young adults after the implementation of universal vaccination in different regions. The data indicate that universal vaccination has significantly reduced the HBsAg positivity rates in Alaska, Taiwan, Singapore, and China, especially among children. However, HBsAg positivity rates tend to increase with age, particularly in Alaska. On the other hand, the anti-HBs positivity rates improve with age, reflecting good long-term immunity. Data from Taiwan and China show that after universal vaccination, the HBsAg positivity rates remain low, especially in children, with almost zero positivity. These results demonstrate the significant effectiveness of universal vaccination in controlling the spread of the Hepatitis B virus (Adopted from Zhao et al., 2020) |
4.2 Global immunization programs
Global immunization programs have made significant strides in reducing the prevalence of HBV infection. According to the World Health Organization (WHO), universal hepatitis B vaccination has been implemented in 189 countries, leading to a dramatic decrease in the prevalence of HBsAg among children under five years of age, from 4.7% in the pre-vaccine era to 1.3% in 2015 (Hutin et al., 2018; Zhao et al., 2020). This success is attributed to the widespread adoption of the hepatitis B vaccine in national immunization schedules, often starting with a birth dose followed by additional doses during infancy (Hutin et al., 2018).
The Global Alliance for Vaccines and Immunization (GAVI) has played a crucial role in supporting the introduction of hepatitis B vaccines in low-income countries. This has resulted in measurable reductions in HBV-related morbidity and mortality. For instance, in Taiwan, the prevalence of chronic HBV infection in children has declined by more than 90% following the implementation of universal vaccination (Shepard et al., 2006).
However, challenges remain in achieving optimal vaccination coverage. In many countries, particularly in sub-Saharan Africa, the timely administration of the birth dose is not routinely practiced, which hampers efforts to prevent MTCT of HBV (Tall et al., 2021). Studies have shown that the addition of a birth dose to the existing vaccination schedule can further reduce HBV transmission, highlighting the need for its integration into routine immunization programs (Tall et al., 2021).
Efforts to overcome social and economic barriers to vaccination are essential for the global control of HBV. The WHO's Global Hepatitis Program provides a framework for countries to develop and implement effective national strategies to combat hepatitis B. This includes ensuring access to vaccines, improving vaccination coverage, and monitoring the impact of immunization programs (Locarnini et al., 2015; Hutin et al., 2018).
While significant progress has been made in the development and implementation of hepatitis B vaccines, continued efforts are needed to address the remaining challenges. Sustainable vaccination programs, improved coverage, and targeted vaccination efforts for high-risk communities are critical to achieving the global elimination of HBV transmission (Shepard et al., 2006; Zanetti et al., 2008; Locarnini et al., 2015; Hutin et al., 2018; Zhao et al., 2020; Pattyn et al., 2021; Tall et al., 2021).
5 Vaccine-Induced Immunity and Challenges
5.1 Immunological response to HBV vaccines
The immunological response to hepatitis B virus (HBV) vaccines is primarily measured by the production of antibodies against the hepatitis B surface antigen (anti-HBs). Effective vaccination is defined by the induction of protective anti-HBs titers, which are indicative of immunity. Studies have shown that the magnitude of the immune response can be influenced by the type of vaccine used. For instance, a tri-antigenic HBV vaccine (TAV) has been demonstrated to elicit significantly higher anti-HBs titers compared to a mono-antigenic HBV vaccine (MAV) (Langley et al., 2020). This higher antibody response is crucial as it is believed to predict the persistence and durability of seroprotection.
In populations such as healthcare students, who are at high risk of exposure, the persistence of humoral immune protection induced by the primary cycle of hepatitis B vaccination has been shown to be high, with a seroprotection prevalence of 73.8% and an anamnestic response rate of 90.9% following a challenge dose (Rahmani et al., 2022). This indicates that the majority of individuals maintain effective immunity for over two decades post-vaccination.
5.2 Factors influencing vaccine efficacy
Several factors can influence the efficacy of HBV vaccines. Age, gender, body mass index (BMI), smoking status, and the presence of concomitant diseases have all been identified as significant determinants of vaccine response. For example, adults aged 40 years and older, males, individuals with a BMI of 25 or higher, smokers, and those with concomitant diseases exhibit significantly decreased responses to the hepatitis B vaccine (Yang et al., 2016). Additionally, the timing of vaccination in relation to disease status is critical. In patients with inflammatory bowel disease (IBD), vaccination during disease remission and before the initiation of immunosuppressive therapy is associated with better immune responses (Jiang et al., 2017).
Host genetic factors also play a role in vaccine-induced immunity. Variations in genes such as IFNG, MAPK8, and IL10RA have been associated with differences in peak anti-HBs levels, which are directly related to the durability of the antibody response and long-term vaccine efficacy (Hennig et al., 2008).
5.3 Overcoming immunological challenges
To overcome the immunological challenges associated with HBV vaccination, several strategies have been proposed. One approach is the use of higher vaccine doses or additional booster doses. For instance, a double dose of the HBV vaccine and a four-dose schedule have been shown to result in better immune responses compared to the standard three-dose regimen, particularly in people living with HIV (PLWH) (Figure 2) (Tian et al., 2021). Similarly, high-dose vaccines have been found to provide more satisfactory long-term immunogenicity in hemodialysis patients (Yao et al., 2021). Another strategy involves the use of adjuvants to enhance the immune response. The use of Fms-like tyrosine kinase 3 ligand (Flt3L) as an adjuvant in DNA vaccines has been shown to significantly enhance both humoral and cellular immune responses, suggesting potential improvements in vaccine efficacy (Zhou et al., 2010).
|
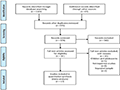
Figure 2 Flowchart of the study selection process (Adapted from Tian et al., 2021) Image caption: This chart illustrates the screening process for a systematic review and meta-analysis. Initially, 1 123 records were identified through database searching, and 27 additional records were identified through other sources. After removing duplicates, 576 records were left for screening. Following the screening, 574 records were excluded, leaving 32 articles for full-text assessment. After full-text assessment, 15 articles were excluded for specific reasons: 5 articles involved children and adolescents, 8 were retrospective studies, and 2 had repeated samples. Ultimately, 17 studies were included in the quantitative synthesis (meta-analysis). This chart clearly shows the entire process from the initial identification of records to the final inclusion in the meta-analysis, detailing each screening stage and the specific reasons for exclusion (Adapted from Tian et al., 2021) |
Therapeutic vaccines are also being developed to induce robust T-cell responses in chronic HBV patients. A novel vaccine formulation comprising particulate hepatitis B surface and core antigens, combined with the ISCOMATRIX™ adjuvant, has demonstrated the ability to break immune tolerance and induce potent T-cell responses in HBV transgenic mice (Buchmann et al., 2013). While HBV vaccines are generally effective, various factors can influence their efficacy. Addressing these challenges through higher doses, additional booster doses, the use of adjuvants, and the development of therapeutic vaccines can enhance the immune response and provide long-term protection against HBV infection.
6 Innovations and Advances in Vaccine Technology
6.1 Recombinant and DNA vaccines
Recombinant DNA technology has revolutionized the development of hepatitis B vaccines. The first recombinant hepatitis B vaccine was produced by expressing the hepatitis B surface antigen (HBsAg) in yeast cells, specifically Saccharomyces cerevisiae. This method allowed for the large-scale production of vaccines without the need for human plasma, thereby reducing the risk of contamination with other pathogens (Zhang et al., 2019). The recombinant vaccine, Engerix-B, has shown excellent immunogenicity and protective efficacy in various populations, including neonates, children, and adults (Keating and Noble, 2003).
Further advancements have led to the development of vaccines that include additional components of the hepatitis B virus, such as the Pre-S1 and Pre-S2 antigens. These components have been shown to enhance the immunogenic response, particularly in populations that are non-responsive to the conventional HBsAg-only vaccines. For instance, the Bio-Hep-B vaccine, which includes Pre-S1 and Pre-S2 antigens, demonstrated significantly higher immunogenicity and a more rapid antibody response compared to Engerix-B in neonates (Yerushalmi, 1997).
DNA vaccines represent another innovative approach. These vaccines involve the direct introduction of plasmid DNA encoding the HBsAg into the host cells, leading to the production of the antigen and subsequent immune response. Preclinical studies have shown that DNA vaccines can induce potent and sustained immune responses, and they have been evaluated in various animal models (Cove, 2014). Clinical trials have also demonstrated the potential of DNA vaccines to elicit protective antibody responses in human subjects who did not respond to conventional vaccines (Rottinghaus et al., 2003).
6.2 Novel delivery methods
The delivery method of a vaccine can significantly impact its efficacy. Traditional intramuscular injections are effective but may not always be the most efficient or practical method, especially in resource-limited settings. Novel delivery methods are being explored to enhance the immunogenicity and ease of administration of hepatitis B vaccines.
One such method is particle-mediated epidermal delivery (PMED), which involves the use of a gene gun to deliver DNA-coated particles directly into the skin. This method has shown promise in eliciting strong immune responses in individuals who were non-responsive to conventional hepatitis B vaccines (Rottinghaus et al., 2003). Another innovative approach is the use of live-attenuated bacteria as carriers for DNA vaccines. For example, a study demonstrated that a DNA vaccine delivered via live-attenuated Salmonella typhimurium induced a stronger cytotoxic T lymphocyte (CTL) response compared to traditional recombinant HBsAg vaccines (Woo et al., 2001).
In vivo electroporation is another technique that has been used to enhance the delivery and efficacy of DNA vaccines. This method involves the application of electrical pulses to increase the permeability of cell membranes, thereby facilitating the uptake of DNA. Preclinical studies have shown that electroporation can significantly enhance the immunogenicity of DNA vaccines in both small and large animal models (Cova, 2014).
6.3 Therapeutic vaccines
While prophylactic vaccines have been highly effective in preventing hepatitis B virus (HBV) infection, therapeutic vaccines are being developed to treat chronic HBV infections. Chronic HBV infection is characterized by weak and functionally impaired virus-specific immune responses, which contribute to the persistence of the virus. Therapeutic vaccines aim to stimulate these immune responses to achieve virus clearance.
DNA-based therapeutic vaccines have shown promise in preclinical studies. These vaccines can induce potent immune responses and have been evaluated in various animal models, including mice, chimpanzees, and woodchucks. Strategies to optimize the efficacy of these vaccines include the use of genetic adjuvants, combination with antiviral drugs, and prime-boost regimens (Cova, 2014).
One innovative therapeutic approach involves the use of mucosal immunization with DNA vaccines. A study demonstrated that an oral mucosal DNA vaccine delivered via live-attenuated Salmonella typhimurium induced a strong CTL response, which is crucial for the clearance of HBV from hepatocytes. This method may be particularly beneficial for chronic HBV carriers, as it minimizes the risk of side effects associated with humoral responses, such as serum sickness and immune complex deposition (Woo et al., 2001).
In conclusion, the advancements in recombinant and DNA vaccine technology, novel delivery methods, and the development of therapeutic vaccines represent significant strides in the fight against hepatitis B. These innovations hold the potential to enhance vaccine efficacy, improve immunogenicity in non-responders, and provide new treatment options for chronic HBV infections (Mcaleer et al., 1984; Yerushalmi, 1997; Yap et al., 1992; Woo et al., 2001; Keating and Noble, 2003; Rottinghaus et al., 2003; Cova, 2014; Zhao et al., 2020).
7 Policy and Implementation Strategies
7.1 Global health policies
The global health policies surrounding hepatitis B virus (HBV) control have been significantly shaped by the World Health Organization (WHO) and other international health bodies. The WHO's Global Hepatitis Program has provided a comprehensive framework for action, which has been instrumental in guiding national responses to HBV control (Locarnini et al., 2015). Universal vaccination programs have been a cornerstone of these policies, with 168 countries implementing such programs by 2019, leading to a substantial decrease in HBV-related morbidity and mortality (Zanetti et al., 2008; Pattyn et al., 2021). The WHO's 2016 global health sector strategy on viral hepatitis aims for a 90% reduction in new chronic hepatitis B cases and a 65% reduction in mortality by 2030, emphasizing the importance of universal birth-dose vaccination and full vaccine coverage (Spearman et al., 2017).
7.2 Regional programs and success stories
Different regions have tailored their HBV vaccination strategies to address local epidemiological patterns and healthcare infrastructure. In Latin America, for instance, the endemicity of HBV varies widely, necessitating diverse prevention strategies. The highest priority in this region has been the prevention of perinatal and early childhood transmission, with significant success in reducing HBV prevalence in children (Oh, 1990). Taiwan's implementation of universal hepatitis B immunization in the 1990s led to a more than 90% decline in chronic infection rates among children, showcasing the effectiveness of early and widespread vaccination efforts (Shepard et al., 2006).
Sub-Saharan Africa faces unique challenges due to high HBV seroprevalence. Despite these challenges, the region has made strides towards the 2030 elimination targets through the universal implementation of the HBV birth-dose vaccine and improved access to affordable diagnostics and antiviral therapy (Gomes et al., 2019). These efforts are crucial for preventing new infections and managing chronic cases, thereby reducing the overall disease burden.
7.3 Recommendations for policy makers
To further advance the global fight against hepatitis B, policymakers must prioritize several key strategies. The universal implementation of the HBV birth-dose vaccine should be mandated and rigorously enforced. This measure is critical for preventing mother-to-child transmission, which is a significant route of chronic HBV infection (Zhao et al., 2020). Additionally, ensuring full vaccine coverage through sustained immunization programs is essential. Policymakers should focus on maintaining high vaccination rates and addressing gaps in coverage, particularly in resource-poor settings (Shepard et al., 2006; Spearman et al., 2017).
Targeted vaccination efforts should be directed towards high-risk groups, including healthcare workers, individuals with high-risk behaviors, and populations in regions with high HBV endemicity. Routine screening and immunization of pregnant women, as well as other at-risk adults, can significantly reduce the transmission and impact of HBV (Alavian et al., 2010). Policymakers should also consider integrating HBV vaccination with other public health initiatives to maximize reach and efficiency.
Improving access to affordable diagnostics and antiviral treatments is vital. Early diagnosis and effective management of chronic HBV infections can prevent severe liver disease and reduce mortality rates. Policymakers should work towards making these healthcare services accessible and affordable, particularly in low-income regions (Spearman et al., 2017). Continuous monitoring and evaluation of vaccination programs are necessary to measure their impact and identify areas for improvement. Policymakers should invest in robust surveillance systems to track HBV infection rates, vaccination coverage, and program outcomes. This data-driven approach will enable timely adjustments to strategies and ensure the sustained success of HBV control efforts (Shepard et al., 2006; Pattyn et al., 2021).
A multifaceted approach that includes universal vaccination, targeted immunization of high-risk groups, improved access to diagnostics and treatment, and continuous program evaluation is essential for the global eradication of hepatitis B. Policymakers must commit to these strategies to achieve the ambitious goals set by the WHO and ultimately render the world hepatitis B free.
8 Future Directions in Global HBV Control
8.1 Innovations in vaccine technology
The future of global HBV control hinges significantly on advancements in vaccine technology. Current vaccines have proven effective, but there is always room for improvement. Innovations such as the development of therapeutic vaccines, which not only prevent infection but also treat existing chronic infections, are on the horizon. These vaccines aim to stimulate the immune system to clear the virus from the body, offering hope for millions of chronic HBV carriers (Locarnini et al., 2015; Pattyn et al., 2021). Additionally, research is ongoing to create vaccines that are more effective in populations with a high prevalence of HBV mutations, which can lead to vaccine escape (Mokaya et al., 2018). The integration of novel adjuvants and delivery systems, such as nanoparticle-based vaccines, could enhance the immunogenicity and efficacy of HBV vaccines, making them more accessible and effective across diverse populations (Zanetti et al., 2008; Pattyn et al., 2021).
8.2 Enhancing global collaboration
Global collaboration is crucial for the eradication of HBV. The World Health Organization (WHO) has already laid the groundwork with its Global Hepatitis Program, which provides a framework for countries to develop their national strategies (Locarnini et al., 2015). However, more needs to be done to foster international cooperation. This includes sharing data and resources, standardizing treatment protocols, and coordinating vaccination campaigns, especially in regions with high HBV prevalence (Kao and Chen, 2002; Shepard et al., 2006). Collaborative efforts should also focus on research and development, pooling resources to accelerate the discovery of new treatments and vaccines. Partnerships between governments, non-governmental organizations, and the private sector can help to mobilize the necessary funding and expertise to tackle HBV on a global scale (Lavanchy, 2005; Nayagam et al., 2016).
8.3 Addressing remaining challenges
Despite significant progress, several challenges remain in the global fight against HBV. One of the primary issues is the disparity in vaccine coverage and healthcare access between high-income and low-income countries. Many resource-poor nations struggle with the implementation of universal vaccination programs due to economic and logistical barriers (Shepard et al., 2006; Zanetti et al., 2008). Additionally, the high prevalence of HBV in certain regions, such as sub-Saharan Africa and parts of Asia, necessitates targeted interventions to reduce transmission rates (Ott et al., 2012; Mokaya et al., 2018). Another challenge is the management of chronic HBV infections, which requires lifelong monitoring and treatment. The development of affordable and accessible antiviral therapies is essential to reduce the burden of chronic HBV and prevent complications such as cirrhosis and hepatocellular carcinoma (Margolis et al., 1991; Kao and Chen, 2002). Finally, addressing the issue of HBV mutations that lead to drug and vaccine resistance is critical. Enhanced diagnostic screening and consistent drug supply are necessary to manage and mitigate the impact of these mutations (Mokaya et al., 2018). The future of global HBV control will depend on continued innovation in vaccine technology, enhanced global collaboration, and addressing the remaining challenges. By focusing on these areas, we can move closer to the goal of eliminating HBV as a major public health threat.
9 Concluding Remarks
The global epidemiology of hepatitis B (HBV) has seen significant changes over the past few decades, primarily due to the widespread implementation of vaccination programs. Universal HBV vaccination has led to a substantial decrease in the prevalence of chronic HBV infections, particularly in children, as evidenced by the dramatic reductions in regions such as Taiwan and South East Asia. Despite these successes, the absolute number of individuals living with chronic HBV has increased, highlighting the need for continued and enhanced vaccination efforts. The introduction of the World Health Organization's Global Hepatitis Program has provided a framework for countries to develop national strategies to combat HBV, which has been crucial in reducing the incidence and prevalence of the disease.
Future research should focus on several key areas to further reduce the global burden of HBV. There is a need for improved strategies to enhance vaccine coverage, particularly in resource-poor regions and among high-risk populations such as older adults and those with comorbid conditions. Research into the mechanisms of vaccine non-responsiveness and the development of more effective vaccines or adjuvants is also critical. Additionally, studies should aim to better understand the epidemiology of HBV at the sub-national level to tailor interventions more effectively. Finally, the integration of HBV vaccination with other public health initiatives, such as the prevention of mother-to-child transmission, should be explored to maximize the impact of vaccination programs.
The fight against hepatitis B has made remarkable progress, but significant challenges remain. The success of vaccination programs in reducing HBV prevalence and related morbidity and mortality underscores the importance of maintaining and expanding these efforts. As we move forward, it is essential to address the gaps in vaccine coverage and effectiveness, particularly among vulnerable populations. By continuing to invest in research and public health initiatives, we can work towards the ultimate goal of eliminating hepatitis B as a global health threat. The lessons learned from the successes and challenges of HBV vaccination programs will be invaluable in guiding future strategies to combat this and other infectious diseases.
Conflict of Interest Disclosure
The author affirms that this research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
Alavian S., Fallahian F., and Lankarani K., 2010, Implementing strategies for hepatitis B vaccination, Saudi Journal of Kidney Diseases and Transplantation, 21(1): 10-22.
Buchmann P., Dembek C., Kuklick L., Jäger C., Tedjokusumo R., Freyend M., Drebber U., Janowicz Z., Melber K., and Protzer U., 2013, A novel therapeutic hepatitis B vaccine induces cellular and humoral immune responses and breaks tolerance in hepatitis B virus (HBV) transgenic mice, Vaccine, 31(8): 1197-203.
https://doi.org/10.1016/j.vaccine.2012.12.074
PMid:23306359
Chen W., and Gluud C., 2005, Vaccines for preventing hepatitis B in health-care workers, The Cochrane Database of Systematic Reviews, (4): CD000100.
https://doi.org/10.1002/14651858.CD000100.PUB3
Cova L., 2014, Advances and challenges in the development of therapeutic DNA vaccines against hepatitis B virus infection, Current Gene Therapy, 14(3): 149-160.
https://doi.org/10.2174/1566523214666140509102644
PMid:24828255
Goldstein S., Zhou F., Hadler S., Bell B., Mast E., and Margolis H., 2005, A mathematical model to estimate global hepatitis B disease burden and vaccination impact, International Journal of Epidemiology, 34(6): 1329-1339.
https://doi.org/10.1093/ije/dyi206
PMid:16249217
Gomes C., Wong R., and Gish R., 2019, Global perspective on hepatitis B virus infections in the era of effective vaccines, Clinics in Liver Disease, 23(3): 383-399.
https://doi.org/10.1016/j.cld.2019.04.001
PMid:31266615
Hennig B.J., Fielding K., Broxholme J., Diatta M., Mendy M., Moore C., Pollard A., Rayco-Solon P., Sirugo G., Sande M., Waight P., Whittle H., Zaman S., Hill A., and Hall A.J., 2008, Host genetic factors and vaccine-induced immunity to hepatitis B Virus Infection, PLoS One, 3(3): e1898.
https://doi.org/10.1371/journal.pone.0001898
PMid:18365030 PMCid:PMC2268746
Hutin Y., Desai S., and Bulterys M., 2018, Preventing hepatitis B virus infection: milestones and targets, Bulletin of the World Health Organization, 96: 443-443.
https://doi.org/10.2471/BLT.18.215210
PMid:29962544 PMCid:PMC6022619
Hwang E., and Cheung R., 2012, Global epidemiology of hepatitis B virus (HBV) infection, North American Journal of Medicine and Science, 4: 7.
https://doi.org/10.7156/V4I1P007
Jiang H., Wang S., Deng M., Li Y., Ling Z., Shao L., and Ruan B., 2017, Immune response to hepatitis B vaccination among people with inflammatory bowel diseases: a systematic review and meta-analysis, Vaccine, 35(20): 2633-2641.
https://doi.org/10.1016/j.vaccine.2017.03.080
PMid:28404358
Kao J., and Chen D., 2002, Global control of hepatitis B virus infection, The Lancet Infectious Diseases, 2(7): 395-403.
https://doi.org/10.1016/S1473-3099(02)00315-8
PMid:12127351
Keating G., and Noble S., 2003, Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B, Drugs, 63(10): 1021-1051.
Langley J., Vesikari T., Machluf N., Spaans J., Yassin-Rajkumar B., Anderson D., Popovic V., and Diaz-Mitoma F., 2020, 8. higher hepatitis B antibody titres induced in all adults vaccinated with a tri-antigenic hepatitis B (HBV) vaccine, compared to a mono-antigenic HBV vaccine: results from two pivotal phase 3 double-blind, randomized studies (PROTECT and CONSTANT), Open Forum Infectious Diseases, 7: S26-S27.
https://doi.org/10.1093/ofid/ofaa439.053
Lavanchy D., 2005, Worldwide epidemiology of HBV infection, disease burden, and vaccine prevention, Journal of Clinical Virology, 34(Suppl 1): S1-S3.
https://doi.org/10.1016/S1386-6532(05)00384-7
PMid:16461208
Lavanchy D., and Kane M., 2016, Global epidemiology of hepatitis B virus infection, Hepatitis B Virus in Human Diseases, 2006: 187-203.
https://doi.org/10.1007/978-3-319-22330-8_9
Locarnini S., Hatzakis A., Chen D., and Lok A., 2015, Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs, Journal of Hepatology, 62(Suppl): S76-S86.
https://doi.org/10.1016/j.jhep.2015.01.018
PMid:25920093
MacLachlan J., and Cowie B., 2015, Hepatitis B virus epidemiology, Cold Spring Harbor Perspectives in Medicine, 5(5): a021410.
https://doi.org/10.1101/cshperspect.a021410.
PMid:25934461 PMCid:PMC4448582
Margolis H., Alter M., and Hadler S., 1991, Hepatitis B: evolving epidemiology and implications for control, Seminars in Liver Disease, 11: 84-92.
https://doi.org/10.1055/s-2008-1040427
PMid:1832236
Mcaleer W., Buynak E., Maigetter R., Wampler D., Miller W., and Hilleman M., 1984, Human hepatitis B vaccine from recombinant yeast, Nature, 307: 178-180.
https://doi.org/10.1038/307178a0
PMid:6318124
Mokaya J., McNaughton A., Hadley M., Beloukas A., Geretti A., Goedhals D., and Matthews P., 2018, A systematic review of hepatitis B virus (HBV) drug and vaccine escape mutations in Africa: a call for urgent action, PLoS Neglected Tropical Diseases, 12(8): e0006629.
https://doi.org/10.1371/journal.pntd.0006629
PMid:30080852 PMCid:PMC6095632
Nayagam S., Thursz M., Sicuri E., Conteh L., Wiktor S., Low-Beer D., and Hallett T., 2016, Requirements for global elimination of hepatitis B: a modelling study, The Lancet Infectious diseases, 16(12): 1399-1408.
https://doi.org/10.1016/S1473-3099(16)30204-3
PMid:27638356
Oh F., 1990, Hepatitis B in Latin America: epidemiological patterns and eradication strategy, the Latin American regional study group, Vaccine, 8(Suppl): S100-S106.
https://doi.org/10.1016/0264-410X(90)90228-E
Ott J., Stevens G., Groeger J., and Wiersma S., 2012, Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity, Vaccine, 30(12): 2212-2229.
https://doi.org/10.1016/j.vaccine.2011.12.116
Pattyn J., Hendrickx G., Vorsters A., and Damme P., 2021, Hepatitis B vaccines, The Journal of Infectious Diseases, 224: S343-S351.
Razavi-Shearer D., Gamkrelidze I., Nguyen M., Chen D., Damme P., Abbas Z., Abdulla M., Rached A., Adda D., Aho I., Akarca U., Hasan F., Lawati F., Naamani K., Al-Ashgar H., Alavian S., Alawadhi S., Albillos A., Al-Busafi S., Aleman S., Alfaleh F., Aljumah A., Anand A., Anh N., Arends J., Arkkila P., Athanasakis K., Bane A., Ben‐Ari Z., Berg T., Bizri A., Blach S., Mello C., Brandon S., Bright B., Bruggmann P., Brunetto M., Buti M., Chan H., Chaudhry A., Chien R., Choi M., Christensen P., Chuang W., Chulanov V., Clausen M., Colombo M., Cornberg M., Cowie B., Craxì A., Croes E., Cuellar D., Cunningham C., Desalegn H., Dražilová S., Duberg A., Egeonu S., El-Sayed M., Estes C., Falconer K., Ferraz M., Ferreira P., Flisiak R., Fraňková S., Gaeta G., García-Samaniego J., Genov J., Gerstoft J., Goldiş A., Gountas I., Gray R., Pessoa M., Hajarizadeh B., Hatzakis A., Hézode C., Himatt S., Hoepelman A., Hrstic I., Hui Y., Husa P., Jahis R., Janjua N., Jarčuška P., Jaroszewicz J., Kaymakoğlu S., Kershenobich D., Kondili L., Konysbekova A., Krajden M., Kristian P., Laleman W., Lao W., Layden J., Lazarus J., Lee M., Liakina V., Lim Y., Loo C., Lukšić B., Malekzadeh R., Malu A., Mamatkulov A., Manns M., Marinho R., Maticic M., Mauss S., Memon M., Correa M., Méndez-Sánchez N., Merat S., Metwally A., Mohamed R., Mokhbat J., Moreno C., Mossong J., Mourad F., Müllhaupt B., Murphy K., Musabaev E., Nawaz A., Nde H., Negro F., Nersesov A., Nguyen V., Njouom R., Ntagirabiri R., Nurmatov Z., Obekpa S., Ocama P., Oguche S., Omede O., Omuemu C., Opare-Sem O., Opio C., Örmeci N., Papatheodoridis G., Pasini K., Pimenov N., Poustchi H., Quang T., Qureshi H., Ramji A., Razavi-Shearer K., Redae B., Reesink H., Ríos C., Rjašková G., Robbins S., Roberts L., Roberts S., Ryder S., Safadi R., Sagalova O., Salupere R., Sanai F., Sánchez-Ávila J., Saraswat V., Sarrazin C., Schmelzer J., Schréter I., Scott J., Seguin-Devaux C., Shah S., Sharara A., Sharma M., Shiha G., Shin T., Sievert W., Sperl J., Stärkel P., Stedman C., Sypsa V., Tacke F., Tan S., Tanaka J., Tomasiewicz K., Urbánek P., Meer A., Vlierberghe H., Vella S., Vince A., Waheed Y., Waked I., Walsh N., Weis N., Wong V., Woodring J., Yaghi C., Yang H., Yang C., Yesmembetov K., Yosry A., Yuen M., Yusuf M., Zeuzem S., and Razavi H., 2018, Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study, The Lancet Gastroenterology and Hepatology, 3(6): 383-403.
Rahmani A., Montecucco A., Vitturi B., Debarbieri N., Dini G., and Durando P., 2022, Long-term effectiveness of hepatitis B vaccination in the protection of healthcare students in highly developed countries: a systematic review and meta-analysis, Vaccines, 10(11): 1841.
https://doi.org/10.3390/vaccines10111841
Rottinghaus S., Poland G., Jacobson R., Barr L., and Roy M., 2003, Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination, Vaccine, 21(31): 4604-4608.
https://doi.org/10.1016/S0264-410X(03)00447-X
Shepard C., Simard E., Finelli L., Fiore A., and Bell B., 2006, Hepatitis B virus infection: epidemiology and vaccination, Epidemiologic Reviews, 28: 112-125.
Spearman C., Spearman C., Afihene M., Ally R., Apica B., Awuku Y., Cunha L., Dusheiko G., Gogela N., Gogela N., Kassianides C., Kew M., Kew M., Lam P., Lesi O., Lohouès-Kouacou M., Mbaye P., Musabeyezu E., Musau B., Os O., Rwegasha J., Scholz B., Shewaye A., Tzeuton C., Sonderup M., and Sonderup M., 2017, Hepatitis B in sub-Saharan Africa: strategies to achieve the 2030 elimination targets, The Lancet, Gastroenterology and Hepatology, 2(12): 900-909.
https://doi.org/10.1016/S2468-1253(17)30295-9
Tall H., Adam P., Tiendrebeogo A., Vincent J., Schaeffer L., Platen C., Fernandes-Pellerin S., Sawadogo F., Bokoum A., Bouda G., Ouattara S., Ouédraogo I., Herrant M., Boucheron P., Sawadogo A., Betsem E., Essoh A., Kaboré L., Ouattara A., Meda N., Hien H., Gosset A., Giles-Vernick T., Boyer S., Kania D., Vray M., and Shimakawa Y., 2021, Impact of Introducing hepatitis B birth dose vaccines into the infant immunization program in burkina faso: study protocol for a stepped wedge cluster randomized Trial (NéoVac Study), Vaccines, 9(6): 583.
https://doi.org/10.3390/vaccines9060583
Te H., and Jensen D., 2010, Epidemiology of hepatitis B and C viruses: a global overview, Clinics in Liver Disease, 14(1): 1-21.
https://doi.org/10.1016/j.cld.2009.11.009.
Tian Y., Hua W., Wu Y., Zhang T., Wang W., Wu H., Guo C., and Huang X., 2021, Immune response to hepatitis B virus vaccine among people living with HIV: a meta-analysis, Frontiers in Immunology, 12: 745541.
https://doi.org/10.3389/fimmu.2021.745541
Woo P., Wong L., Zheng B., and Yuen K., 2001, Unique immunogenicity of hepatitis B virus DNA vaccine presented by live-attenuated Salmonella typhimurium, Vaccine, 19(20-22): 2945-2954.
https://doi.org/10.1016/S0264-410X(00)00530-2
Yang S., Tian G., Cui Y., Ding C., Deng M., Yu C., Xu K., Ren J., Yao J., Li Y., Cao Q., Chen P., Xie T., Wang C., Wang B., Mao C., Ruan B., Jiang T., and Li L., 2016, Factors influencing immunologic response to hepatitis B vaccine in adults, Scientific Reports, 6(1): 27251.
https://doi.org/10.1038/srep27251
Yao T., Shao Z., Wu L., Dong S., Gao L., Wu Y., Shi X., Shi J., Liu G., Wang J., Zhao H., Guo H., Liu H., Wu X., Liu L., Song X., Zhu J., Zhang Y., Feng Y., Liang X., and Wang S., 2021, Long-term persistent immunogenicity after successful standard and triple-dosed hepatitis B vaccine in hemodialysis patients: a 3-year follow-up study in China, Vaccine, 39(18): 2537-2544.
https://doi.org/10.1016/j.vaccine.2021.03.074
Yap I., Guan R., and Chan S., 1992, Recombinant DNA hepatitis B vaccine containing Pre-S components of the HBV coat protein-a preliminary study on immunogenicity, Vaccine, 10(7): 439-442.
https://doi.org/10.1016/0264-410X(92)90391-V
Yerushalmi B., Raz R., Blondheim O., Shumov E., Koren R., and Dagan R., 1997, Safety and immunogenicity of a novel mammalian cell-derived recombinant hepatitis B vaccine containing Pre-S1 and Pre-S2 antigens in neonates, The Pediatric Infectious Disease Journal, 16(6): 587-592.
.https://doi.org/10.1097/00006454-199706000-00009.
PMid:9194109
Zanetti A., Damme P., and Shouval D., 2008, The global impact of vaccination against hepatitis B: a historical overview, Vaccine, 26(49): 6266-6273.
https://doi.org/10.1016/j.vaccine.2008.09.056.
Zhang Z., Liang Z., Zeng J., Zhang J., He P., Su J., Zeng Y., Fan R., Zhao D., Ma W., Zeng G., Zhang Q., and Zheng H., 2019, Immunogenicity and safety of an inactivated enterovirus 71 vaccine administered simultaneously with recombinant hepatitis B vaccine and group A meningococcal polysaccharide vaccine: a phase IV, open-label, single-center, randomized, non-inferiority trial, The Journal of Infectious Diseases, 220(3): 392-399.
https://doi.org/10.1093/infdis/jiz129.
Zhao H., Zhou X., and Zhou Y., 2020, Hepatitis B vaccine development and implementation, Human Vaccines and Immunotherapeutics, 16: 1533-1544.
https://doi.org/10.1080/21645515.2020.1732166
Zhou Q., Wang F., Yang F., Wang Y., Zhang X., and Sun S., 2010, Augmented humoral and cellular immune response of hepatitis B virus DNA vaccine by micro-needle vaccination using Flt3L as an adjuvant, Vaccine, 28(5): 1357-1362.
https://doi.org/10.1016/j.vaccine.2009.11.006
PMid:19932674
(1).png)
. FPDF(win)
. FPDF(mac)
. HTML
. Online fPDF
Associated material
. Readers' comments
Other articles by authors
. Weimin Sun
Related articles
. Hepatitis B virus (HBV)
. Vaccination
. Epidemiology
. Hepatocellular carcinoma (HCC)
. Global health strategies
Tools
. Post a comment